Optimizing Salesforce Health Cloud for Health and Life Sciences Organizations
How Digital Validation and Test Automation Provide a Competitive Edge
As organizations look for ways to incorporate technological advancements to remain competitive, many within the healthcare and life sciences (HLS) industry have turned to Salesforce Health Cloud to enhance their day-to-day operations. This integration of advanced technologies into healthcare, particularly in the context of regulated data, demands a rigorous approach to compliance and quality assurance to meet industry regulations and customer expectations.
Salesforce Health Cloud holds the promise of enhancing the quality of HLS operations. This platform provides tools for healthcare providers to manage patient data, track patient interactions, and collaborate with care teams. It enables healthcare professionals to have a 360-degree view of each patient's health information from various sources such as medical history, treatment plans, and communication records.
While Salesforce Health Cloud has the capability to enhance patient safety, streamline regulatory compliance, and ensure the reliability of healthcare systems, this capability is further enhanced through the implementation of test automation and digital validation.
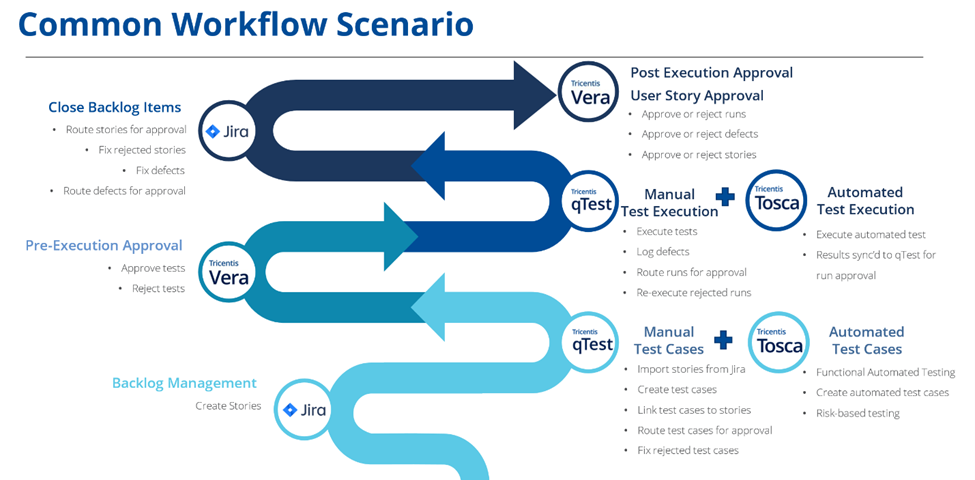
Validation of Salesforce Health Cloud for medical devices can be challenging to complete using traditional paper-based validation methods due to the vast amount of information healthcare providers need to keep track of. Maintaining data integrity and generally managing data becomes increasingly difficult overtime. While using a document management system speeds up this process, there are still paper-based activities such as “document approval” that can be broken down in incremental steps allowing organizations to shift left the approvals happening when the decisions are made.
Implementing a digital validation process covering requirements testing and issue management provides a robust path for development and testing to meet compliance, while providing accurate metrics, detailed process flows, and next steps. It can also reduce the need for paper and document management systems.
In the life sciences sector, Salesforce Health Cloud is used for managing complex processes such as patient drug reactions, nurse assessments, and patient interaction. Digital validation and Salesforce Health Cloud testing ensures that these critical processes are executed accurately and in accordance with regulatory requirements, such as Good Manufacturing Practices (GMP) and Good Clinical Practices (GCP).
Automated testing tools such as Tricentis Tosca can validate the functionality of Salesforce Health Cloud modules that handle sensitive data, ensuring the integrity and security of information crucial for compliance. The ability of digital validation to create comprehensive and repeatable test scenarios is particularly crucial in the life sciences, where precision and reliability are paramount.
By implementing digital validation for Salesforce Health Cloud with test automation organizations can formally review and approve tests (manual and automated) pre and post execution in an integrated solution that manages the end to end validation process. The result is enhanced operational efficiency, reduced risk of compliance errors, and accelerated time-to-market for life sciences products and services. Not only does this ultimately contribute to the overall success and compliance of organizations in this highly regulated industry; it also allows end-users (such as patients, medical teams, and so on) access to potentially life-saving products and services at a faster rate.
While digital validation ensures compliance and increases accuracy and speed of compliance-based activities, test automation accelerates the testing process and increases test coverage. This allows companies within the Healthcare and Life Sciences space to efficiently validate various scenarios. Automated test scripts can be created to verify critical business processes, reducing the time and effort required for testing while improving accuracy. Automated testing can also help to streamline your regression testing process by executing tests unattended expanding your testing volume.
Testing Salesforce Health Cloud for medical device applications involves a comprehensive approach to ensure the reliability, compliance, and security of the integrated solution. Utilizing Tricentis Tosca for your automated testing simplifies this process, but there is still a need for various forms of testing, including:
- Integration Testing: This type of testing verifies that different components or systems work together as intended. In the context of Salesforce Health Cloud, this may involve testing integrations with other Salesforce products, third-party applications, or external systems.
- End-to-End Testing: This type of testing simulates real-world scenarios and ensures that the entire application, including the user interface and underlying processes, functions correctly.
- Regression Testing: After changes or updates are made to the Salesforce Health Cloud environment, regression testing helps ensure that existing functionalities still work as expected.
Choosing the right technology platform to support your business can be challenging. TTC Global’s broad experience working with a multitude of industry leading applications enables us to provide optimized solutions best suited to your organization’s needs. Leveraging our team’s knowledge of automated technologies and testing expertise, we collaborate with you and our providers to improve testing efficiencies, software quality, and customer value delivered. TTC helps to ensure the right technology fit to meet your data validation and test automation needs.
About TTC Global
TTC Global understands the Health and Life Sciences Industry's need for speed to market, while meeting multiple regulatory compliance requirements including 21 CFR Part 11, GxP Compliance, IEC 62304 and ISO 13485, while having extensive experience testing SAP. TTC Global can help modernize the quality management system incorporating new technologies and providing big data analytics while still meeting compliance in a rigorous regulatory landscape. TTC Global applies a risk-based approach to testing, allowing organizations to become more efficient, innovate and shift left, while ensuring the security of patient data and compliance.




